Article originaly posted by Chan Zuckerberg Science Initiative on Medium
Researchers Work to Better Understand the Role of Inflammation in Disease
Inflammation is a natural defense that helps our bodies maintain a healthy state, but chronic inflammation results in harmful diseases like asthma, arthritis, and heart disease. Knowing more about inflammation will increase our understanding of many diseases and improve our ability to cure, prevent, or manage them.
We’re supporting 29 teams of scientists, engineers, and clinicians to study the role of inflammation in maintaining health and triggering diseases. Learn about CZI’s inflammation grantees and the insights they’re uncovering:
Investigating Similarities and Difference Between Chronic and Acute Inflammation
Using emerging and powerful methods of analyzing single cells, researchers aim to discover commonalities between different types of inflammation. Three young scientists are collaborating to clarify how specific cells associated with metabolism, the immune system, and the nervous system sense and respond to chronic or acute inflammation. This project brings together expertise from neurobiology and behavior, intestinal biology and the microbiota, and inflammation and metabolism to pioneer a holistic approach to studying inflammation.
“A lens into the evolution of inflammation is what most excites me. We hope to uncover fundamental biological processes that contribute to health and that go awry in chronic conditions such as obesity.” — Marcelo Dietrich, Yale University
Understanding How Age Influences Susceptibility to Coronavirus Infection
As we get older, we become more susceptible to infection, especially to respiratory diseases. Two scientists in Texas are exploring what changes as we age, and how we can use this information to improve disease outcomes in elderly patients. This team will use a mouse model to investigate how coronaviruses such as SARS, MERS, and SARS-CoV2 cause more severe effects in the elderly.
“When I go to the doctor with a respiratory infection, I am treated the same as my son and my dad, despite the fact our different ages render very different disease and immune responses. We are targeting individual cells and seeking to define the molecular mechanisms of age-dependent respiratory disease following virus infection.” — Vineet Menachery, University of Texas Medical Branch at Galveston
Imaging Gut Immune Cells and Microbes to Understand Health and Disease
High-resolution microscopes allow scientists to watch biological processes in real time. Scientists at Stanford are using a creative combination of tools to introduce human microbes into the gut of flies, followed by high-resolution microscopy to record the interactions in these living organisms. This research into the microbiome will help illuminate how cells and microbes deep within our bodies relate to each other and how the microbiome changes the behavior of cells with tumors or other diseases.
“Collaboration is at the heart of how we grow as scientists. It often takes a village to solve a scientific problem, and the harder the problem, the more expertise we require. The O’Brien lab takes a top-down view to understanding fly development, while the Huang lab builds up from single-cell analyses.” — Kerwyn Casey Huang, Stanford University
High-Resolution Profiling of Body Fat Immune Cells in Brazilian Populations
Obesity is an inflammatory disease — it’s also a risk factor for other diseases, including COVID-19. Researchers in Brazil are partnering with surgeons, computational biologists, and immunologists to identify different cell types in the fat tissue of lean and obese Brazilians, which could provide clues to the kinds of cells that may be important in obesity-related complications. This work aligns with other CZI-supported efforts to map body fat tissue from diverse populations around the world, including those in Germany, Israel, and New Zealand.
“Understanding better how inflammation is associated with a given disease paves the way to identify new treatment strategies.” — Mariana Boroni, Brazilian National Cancer Institute
Single-Cell Approaches to Decipher Central Nervous System Trauma
Spinal cord injuries affect hundreds of thousands of people worldwide each year, with devastating consequences. This team will provide novel insights into central nervous system injuries that can help inform future therapies. By building single-cell biology tools, they hope to understand specific cell types that engulf dying cells and debris during injury.
“We’re exploring what cells are responsible for cleaning up the ‘mess’ left after spinal cord injury. Using new and unique tools, we will be able to precisely characterize the ‘cleaners’ in the injured spinal cord, and this information may help us — and others — develop therapies for spinal cord and brain injuries.” — Jony Kipnis, University of Virginia
Understanding Organism-Wide Inflammation After Heart Injury
After a heart attack, human hearts often suffer permanent damage, in part because of inflammatory processes like fibrosis. However, in human infants and in some animals, hearts can be fully repaired following heart attacks. The goals of this project are to determine how heart injury impacts inflammation outside of the heart using models of mice and zebrafish.
“Understanding how the inflammatory response to heart injury differs between fish and mammals may help us better deal with heart attacks in the future. As a physician and a researcher, my goal is to contribute to patient health in both the clinic and the lab.” — James Lo, Weill Cornell Medicine
How Western Lifestyles Modify Immune Responses in East African Populations

Non-communicable diseases are a leading cause of death worldwide, with high prevalence in western, industrialized societies. How do the environmental and genetic factors unique to each person contribute to variation in disease-related traits? A team of researchers is studying how western lifestyles modify inflammatory response at the single-cell level in East African populations, specifically the Turkana people of Kenya.
Lead scientist Julien Ayroles said he realized that most of the genetic research focused on the emergence of non-communicable diseases targets white European populations, which led him to seek out underrepresented populations that could benefit from similar research on the relationships between non-communicable diseases and inflammation.
“We have been working diligently to nurture genuine relationships with tribal leaders and study participants to make sure they understand all aspects of our work, and also with local academic partners to make sure that after this project is done, this line of work continues with local PI and students.
From orchestrating complex field work in remote parts of northern Kenya, to doing advance single-cell genomics in the lab, to working on cutting-edge immunological readouts — no single lab could make this happen. Collaborative science is more rewarding and a lot more fun.” — Julien Ayroles, Princeton University
Understanding the Impact of Urbanization on Immune Systems in Rural and Urban Africa

Nearly 70 percent of the world’s population is expected to live in urban areas by 2050. As this transition accelerates, we need more information about how urbanization contributes to inflammatory diseases, especially in countries in Africa and Asia that are rapidly urbanizing.
This team of researchers will use mass cytometry and single-cell RNA sequencing to profile peripheral blood mononuclear cells (PBMCs) from subjects residing in rural, semi-urban, and urban areas of Africa to study how urbanization impacts the immune systems of these different populations. They will also develop large datasets and computational and visualization tools to contribute to the ongoing global Human Cell Atlas effort.
“We hope to understand the immune system and how it functions in populations living in widely different environments. Understanding this in great detail will help to boost poor vaccine responses in rural areas and help find innovative methods to stop inflammatory diseases in urban centers.” — Maria Yazdanbakhsh, Leiden University Medical Center
Tools to Manipulate Gene Expression in 3D Models of Human Disease
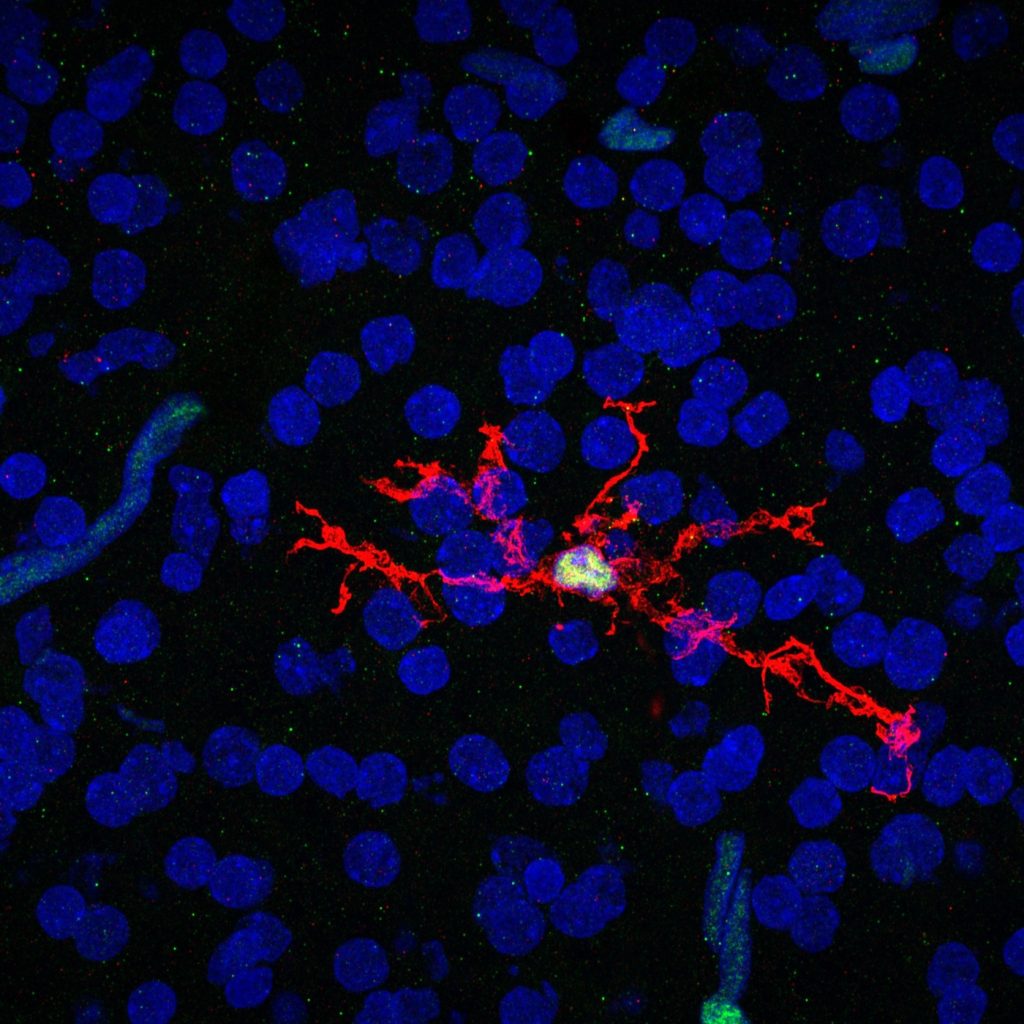
Single-cell biology is able to characterize complex disease at new levels of detail. However, altering many single cells at one time is difficult. This project will develop new methods to manipulate genes predicted to have important roles in neuro-immune diseases. This will allow scalable analysis and manipulation of many genes, along with the flexibility to analyze them in organisms and engineered models of human tissues. Scientists will use these tools to study immune cells in the brain that respond to damage caused by stroke.
“I’m excited at the prospect of modeling human microglia, or the inflammatory cells of the brain, in vivo and using a combination of techniques including CRISPR modifications, CRISPR libraries, and next generation sequencing to understand how human microglia interact with the brain during development, aging, and disease.” — Nicole Coufal, University of California, San Diego
Decoding Inter-Organ Inflammatory Signaling at the Single-Cell Level

Many inflammatory conditions start acutely through damage or infection, yet quickly spread to other organs. The process by which inflammation propagates through an organism is difficult to capture and understand if you study only a single organ. This team will develop a method to capture the exact state of single cells, including where the cells are positioned, for a whole animal. This method will clarify how an inflammatory signal — in this case sepsis — spreads, and what individual cells are responsible for sending and receiving information within tissues. Researchers will use the resulting data to model and simulate sepsis.
“Bringing different disciplines together, such as immunology, data science and engineering in the case of our team, is paramount to building new tools that can’t be created by any one team alone.” — Nicolas Chevrier, The University of Chicago
Mapping Skin Inflammation in Rare Disease Patients
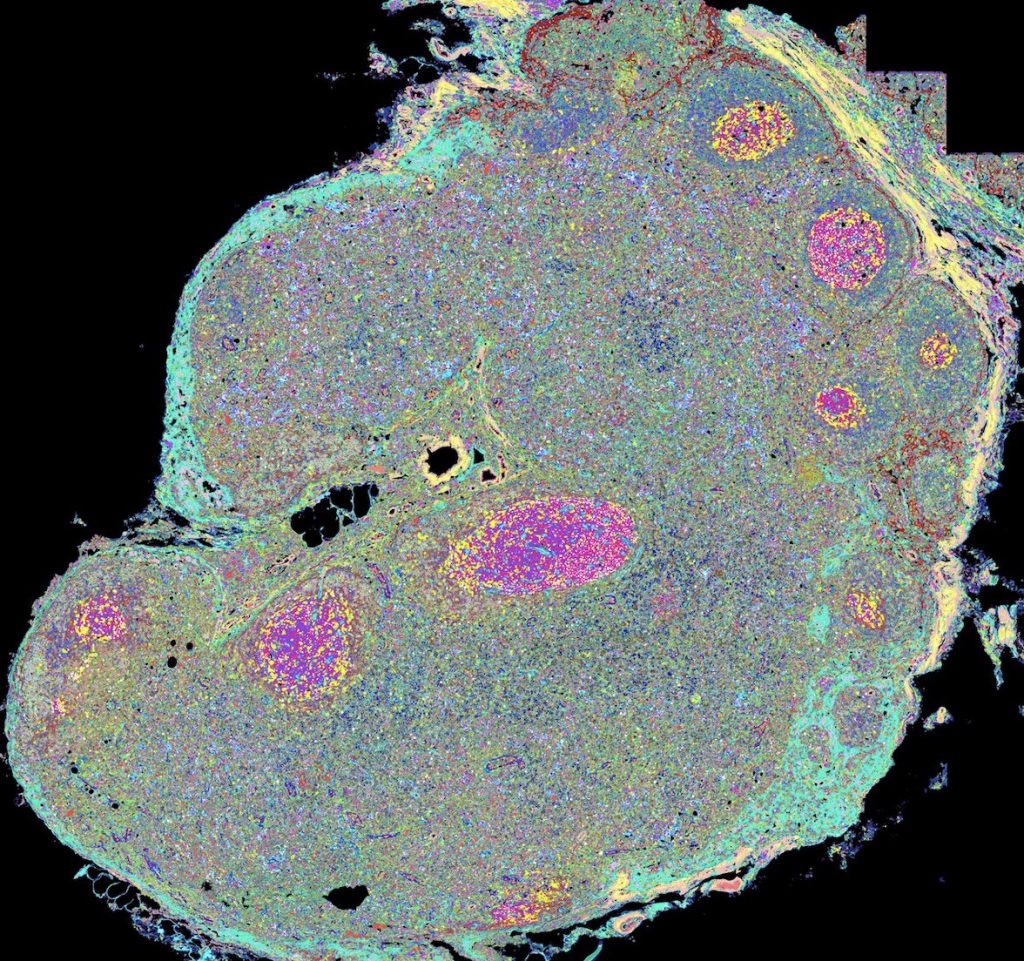
Researchers at the National Institutes of Health are studying skin samples from rare disease patients to examine how the same initial skin cell types result in very different skin diseases over time. Using advanced technology and computational approaches, this project aims to map the cellular, molecular, and spatial networks underlying skin inflammation in the hopes of transforming these basic findings into future clinical impact.
“We’re studying why immune responses differ among individuals and populations — for example, as the COVID-19 pandemic illustrates, the immune response to the virus can differ widely from person to person. This project delves into inflammatory and immune processes in skin tissue to understand responses to disease and vaccination.” — John Tsang, National Institute of Allergy and Infectious Diseases, National Institutes of Health
Mapping Human Inflammatory Responses in Genetically Diverse Populations
The coronavirus pandemic highlights the critical need for effective vaccines that prevent the spread of pathogens. Vaccinations work by sending an inflammatory signal to help prime and train the immune system for a lasting response. This stimulus comes from co-administration of adjuvants, or compounds that elicit an immune response.
Key questions remain on how adjuvants alert the immune system in humans, how they help drive protective immunity, and how responses to different adjuvants vary across ethnic populations. This team will induce inflammation in small samples of human immune tissue from individuals of European, African, and Asian ethnicities to inform more inclusive global vaccine design and development.
“We aim to map the dynamics of early inflammation to help improve drug development and vaccination strategies to combat emerging infectious diseases. Critically, we will also investigate the inter-ethnic differences in the inflammatory response; these have been poorly studied to date but are key when aiming to prevent the spreading of disease on a global scale.” -Calliope Dendrou, University of Oxford